Nature Publication Highlights the Benefits of Tropis® Intradermal When Used to Administer a First-in-Class Self-Amplifying m
GOLDEN, Colo. , April 24 /Businesswire/ - PharmaJet®, a company that strives to improve the performance and outcomes of medicines with its innovative delivery systems, today announced the Nature publication1 of Gennova Biopharmaceutical’s Phase 2/3 clinical trial conducted to evaluate the safety and immunogenicity of its novel samRNA-based Covid-19 vaccine booster. The results demonstrated that GEMCOVAC-OM, administered exclusively with Tropis, is well-tolerated with no related serious adverse events and significantly boosts immune responses against the Omicron variant. Furthermore, the publication cited that the self-amplifying, thermostable mRNA platform delivered intradermally with Tropis provides a framework for next-generation vaccines that can improve accessibility and global equity.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240424868674/en/
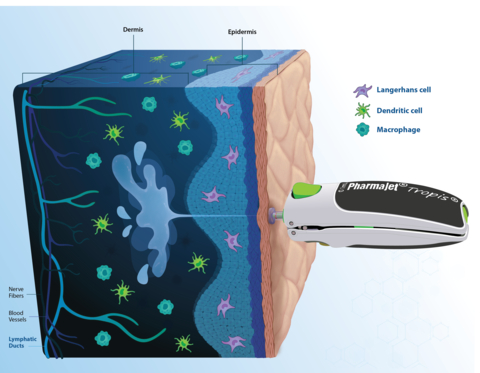
The PharmaJet pioneering technology unlocks the rich potential of the human dermis, paving the way for enhanced immune responses. (Graphic: Business Wire)
ID delivery is known to have the potential to improve the performance of vaccines, but this study is the first time a samRNA vaccine has been developed with a lipid nano-emulsion, and the data show that ID administration of this vaccine is safe and well-tolerated. Tropis unlocks the rich potential of the human dermis, paving the way for enhanced immune responses. Vaccine delivery directly into the dermis enables access to the immune system, with benefits on durability and breadth of immune response, and mucosal immunity. Tropis also enables access to immune cell populations more directly thus creating the potential for dose sparing.
PharmaJet has partnered with Gennova to improve the performance and outcomes of their samRNA platform with PharmaJet’s breakthrough ID delivery technology. The outcome of this collaboration is a needle-free self-amplifying vaccine with advantages over the other mRNA vaccines approved for COVID-19:
- GEMCOVAC-OM is a lyophilized vaccine, stable at 2-8°C, which means it can be distributed through the existing refrigeration supply chain. The booster vaccine is exclusively administered intradermally with Tropis.
- Tropis leverages the rich network of dendritic cells, macrophages, and T cells in the dermal layer providing a more potent and broader immunogenic response than vaccinating into the muscle. Tropis is prequalified by the WHO and approved by numerous regulatory bodies globally. As a needle-free delivery System, Tropis eliminates the need for sharps disposal and needle-stick injuries, as well as increases coverage due to its high acceptability among caregivers and healthcare workers.
“We congratulate our partner Gennova for their compelling data published in this prestigious journal,” said Chris Cappello, President, and Chief Executive Officer, PharmaJet. “This new data adds to the evidence base indicating Tropis needle-free ID administration is an enabler for vaccine platforms.”
Tropis ID (for intradermal administration) and Stratis® SC/IM (for intramuscular and subcutaneous administration) are the only commercially scaled needle-free technologies that enhance the performance of several vaccines and therapeutics. PharmaJet has over 80 global development partners and Tropis has been used to perform over 10 million vaccinations in several countries.
For more information about PharmaJet visit https://pharmajet.com.
Refer to Instructions for Use to ensure safe injections and to review risks.
About PharmaJet
The PharmaJet mission is to improve the performance and outcomes of medicines with our innovative delivery systems that better activate the immune system. We are committed to helping our partners realize their research and commercialization goals while making an impact on public health. PharmaJet Precision Delivery Systems™ can improve increased vaccine effectiveness, allow for a preferred patient and caregiver experience, and offer a proven path to commercialization. They are also safe, fast, and easy-to-use. The Stratis® System has U.S. FDA 510(k) marketing clearance, CE Mark, and WHO PQS certification to deliver medications and vaccines either intramuscularly or subcutaneously. The Tropis® System has CE Mark and WHO PQS certification for intradermal injections. They are both commercially available for global immunization programs. For more information or if you are interested in partnering with PharmaJet to improve the impact of your novel development program, visit https://pharmajet.com or contact PharmaJet here. Follow us on LinkedIn.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240424868674/en/










